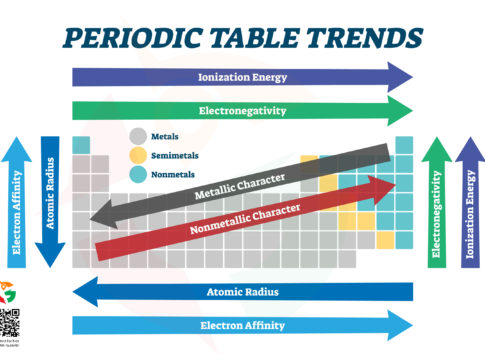
Welcome, young scientists and curious minds, to the first part of our electrifying journey into the heart of chemistry: the Periodic Table! This isn’t just any table; it’s a treasure map that reveals the secrets of everything in the universe, from the air we breathe to the stars in the sky. Let’s dive in and discover how to navigate this fascinating landscape of elements.
The Land of Metals, Semimetals, and Nonmetals
Imagine the Periodic Table as an enormous continent where different regions are home to various elements. On the left side, we have the vast kingdom of Metals. These are the shiny, conductive elements like gold (Au), silver (Ag), and iron (Fe) that can be hammered into shapes and conduct electricity like champions.
Moving towards the right, we enter the mysterious territory of Semimetals (or Metalloids). This region is where elements have a mixed identity, showing both metallic and non-metallic properties. Silicon (Si) and Boron (B) live here, which is crucial for making computer chips and rocket fuel.
On the far right, we reach the realm of Nonmetals. These elements, like oxygen (O) and nitrogen (N), are the building blocks of life. They’re not shiny or good at conducting electricity, but they’re masters of forming compounds that make up the air, water, and living organisms.
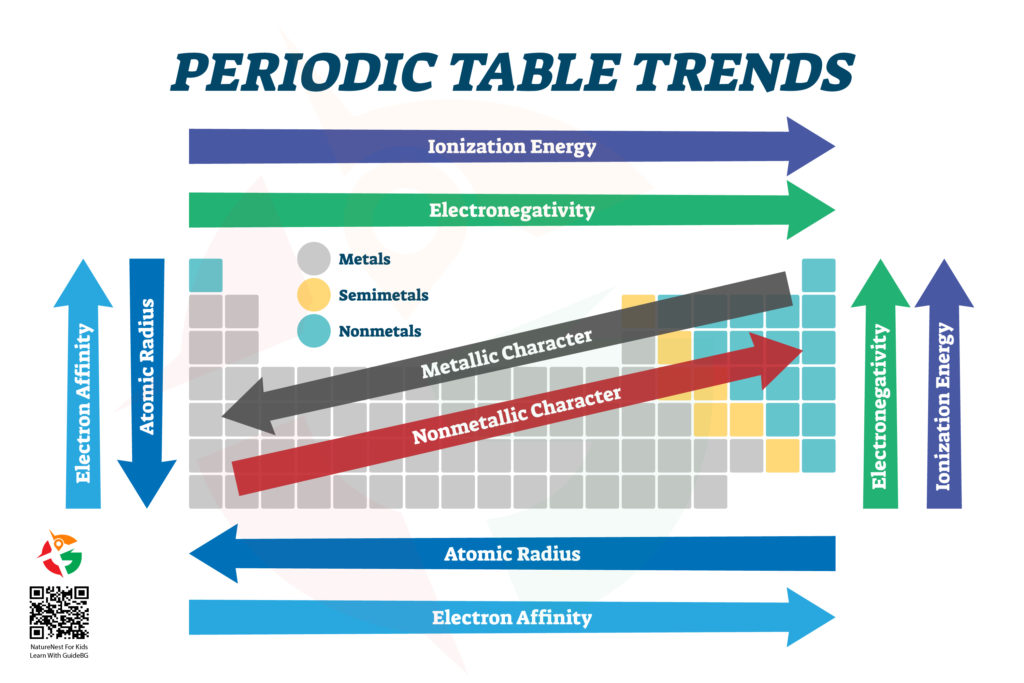
The Quest for Size: Atomic Radius
As we journey across the table, one of the treasures we’re seeking is the atomic radius – basically, how big an atom is. Starting from the left and moving to the right, atoms tend to get smaller. Why? Because they gain more protons, pulling electrons closer and making the atom more compact.
But if you head down any column, atoms get bigger. Each step down adds a new layer of electrons, making the atom’s “orbit” larger. So, Francium (Fr) at the bottom left corner of the table is one of the biggest atoms, while Helium (He) up at the top right is one of the smallest.
The Attraction Adventure: Electron Affinity and Electronegativity
Electron affinity is like how much an atom wants to gain an electron, and as we move from left to right across the table, this desire gets stronger. Chlorine (Cl), for example, is like an electron magnet, eager to snatch up an extra one.
Electronegativity is a bit similar – it’s how strongly an atom tugs on electrons when it’s in a tug-of-war with another atom. The closer you get to Fluorine (F) in the top right, the stronger this pull becomes. Fluorine is like the champion arm-wrestler of electronegativity, defeating all other elements in its grip on electrons.
The Power Play: Ionization Energy
Ionization energy is needed to take an electron away from an atom. This energy increases as you move up and to the right of the table. It’s like trying to steal a toy from a sibling; the closer you get to Helium (He) or Neon (Ne), the harder they will fight to keep their electrons. Atoms in these areas are like the universe’s misers, holding onto their electrons tightly.
Navigating the Table: A Quick Recap
- Metals shine bright on the left, masters of shape-shifting and conducting.
- Semimetals walk the line in the middle, part metal, part mystery.
- Nonmetals rule the right, essential for life and the air we breathe.
- Atomic Radius shrinks as we move right but grows as we dive deeper.
- Electron Affinity and Electronegativity ramp up as we adventure right, with atoms more eager for electron companions.
- Ionization Energy climbs as we ascend and edge right, revealing atoms more reluctant to part with their electrons.
And there you have it, the first part of our guide to the Periodic Table! You’re now equipped to start exploring this incredible landscape of elements independently. Stay tuned for the next part of our adventure, where we’ll dive even deeper into the mysteries of the table. Keep your curiosity burning bright, and who knows what secrets you’ll unlock next on your journey through the world of chemistry.
Stay tuned for our next adventure, where we’ll dive deeper into the atomic arena and explore the heroes and legends that make up the heart of the Periodic Table’s mysteries!