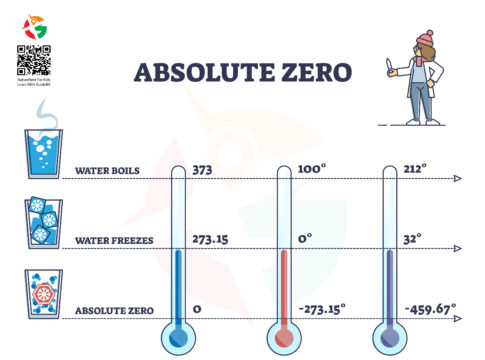
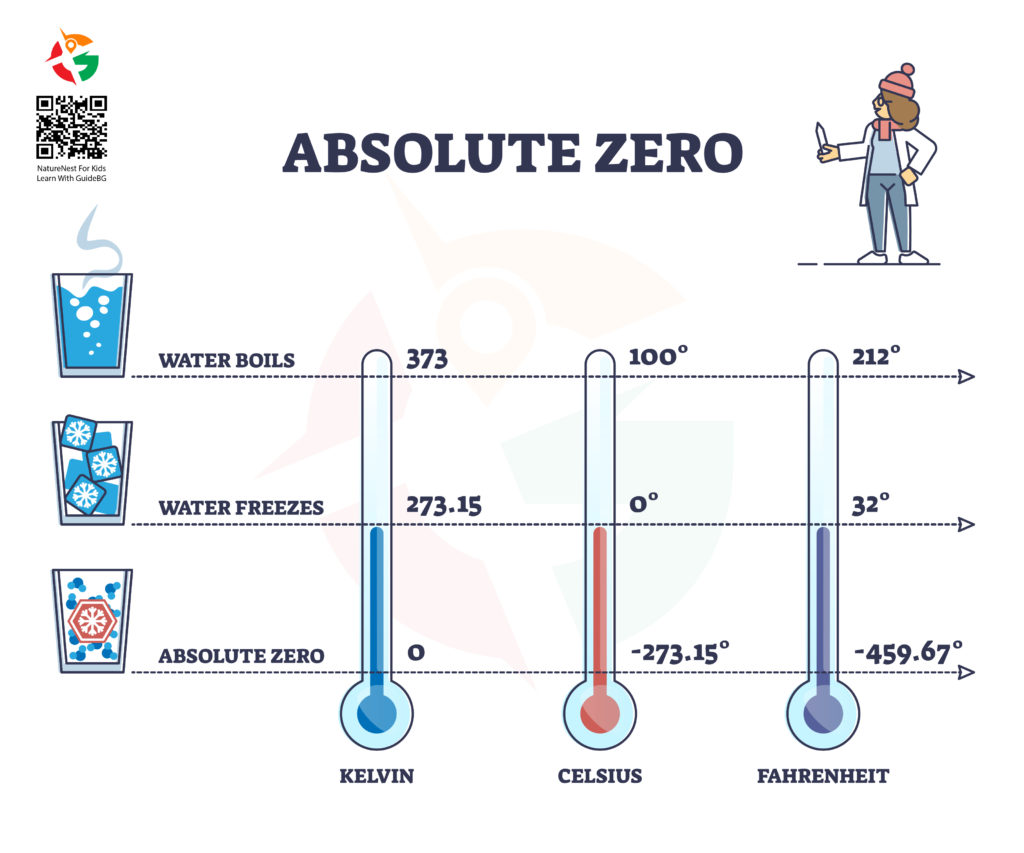
Imagine a world so cold that all motion stops, a temperature where the particles that make up the universe stand still. This isn’t a scene from a science fiction novel; it’s a scientific reality known as absolute zero. Absolute zero is not just a theoretical curiosity. It’s a foundational concept in physics that has profound implications for our understanding of the universe. Let’s dive into the chilling details of absolute zero, exploring its significance, origins, discovery, and impact on past and present scientific endeavors.
What is Absolute Zero?
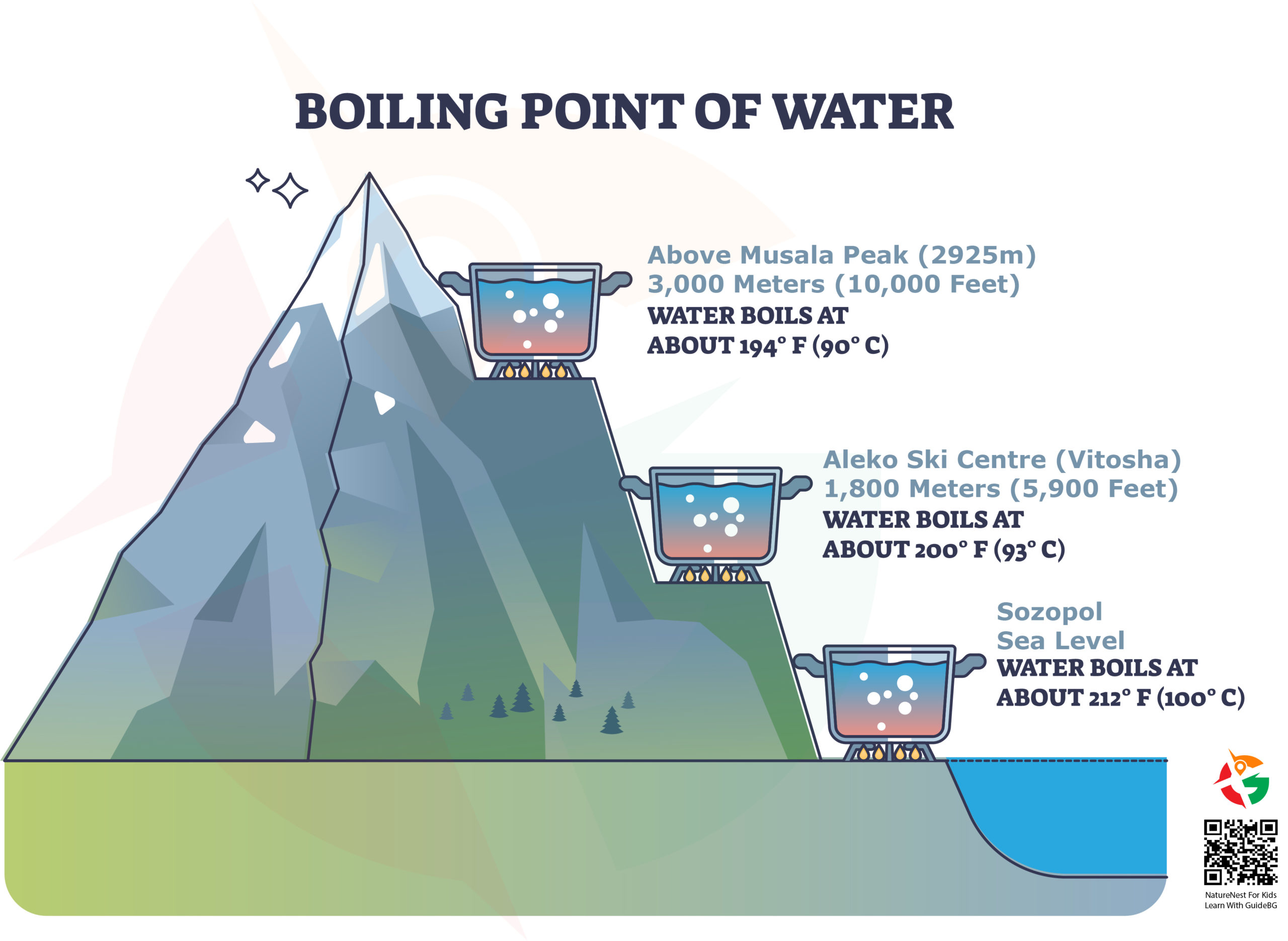
Absolute zero represents the lowest possible temperature where nothing could be colder, and no heat energy remains in a substance. It is the point at which the particles of matter have minimal vibrational motion, essentially coming as close as possible to a complete standstill. In terms of specific numbers, absolute zero is 0 Kelvin (K), which translates to -273.15 degrees Celsius (°C) or -459.67 degrees Fahrenheit (°F). To put this in perspective, water freezes at 0°C (32°F) and boils at 100°C (212°F) at sea level, though boiling points drop at higher altitudes due to decreased air pressure.
The Quest for the Coldest Temperature
The concept of absolute zero emerged from scientists seeking to understand the relationship between temperature and volume in gases. The French physicist Guillaume Amontons, who, in the early 1700s, first hinted at the existence of the lowest possible temperature when he noticed that gases lose volume as they cool. However, Lord Kelvin (William Thomson) in the 19th century defined absolute zero more precisely in terms of the Kelvin scale, a temperature scale that starts at absolute zero.
Why is Absolute Zero Important?
Understanding absolute zero has unlocked vast areas of scientific inquiry and technological advancement. It underpins the laws of thermodynamics, which govern how energy moves and transforms. Moreover, the quest to reach temperatures as close as possible to absolute zero has led to the development of superconductors. These materials conduct electricity with no resistance at very low temperatures. These materials have applications ranging from MRI machines to maglev trains, showcasing the practical benefits of this seemingly abstract concept.
Reaching for the Unreachable
As the Third Law of Thermodynamics explains, hitting absolute zero is out of reach, it would take endless energy to cool anything to absolute zero. Yet scientists have nearly made it. Laser and magnetic cooling methods have chilled atoms to just a hair above absolute zero. This has opened doors to exploring quantum mechanical phenomena, which emerge only in these icy conditions.
Absolute Zero and Quantum Mechanics
Exploring particles near absolute zero has dramatically enhanced our grasp of quantum mechanics. This area of physics dives into particles’ strange, often counterintuitive behavior at minuscule scales. Such studies have paved the way for quantum computers. These advanced machines promise a computing revolution, tackling challenges that today’s technology can’t handle.
Absolute Zero in the Universe
Interestingly, Space offers a natural laboratory for studying phenomena near zero. The cosmic microwave background radiation, the afterglow of the Big Bang, permeates the universe at a temperature of just 2.7 K, providing scientists with a cosmic baseline for studying astrophysical processes.
Absolute zero isn’t just a temperature milestone – it’s a key to unlocking the universe’s secrets. It connects the vastness of space to the tiniest quantum particles, shedding light on matter and energy’s essence. Diving into materials at these ultra-low temperatures, we’re shaping the future of technology and science. Absolute zero broadens our understanding of what’s possible and expands our knowledge of the universe.
Learn how to convert temperature from Fahrenheit to Celsius.